Lab Compliance Management Software
Organise your quality system with our laboratory document management software,in a precise and organised manner and manage easily the accreditation of your laboratory to ISO or equivalent quality standard in your country. Zendo Lims, as a laboratory compliance software, is verified and works with a wide range of laboratories in different sectors that are accredited to quality standards.
* Fully operational demo for 14 days.
ISO LIMS: Ensuring Standards Compliance Worldwide
Our experience, backed by the trust of more than 600 laboratories, will be reflected on your project.




How Our LIMS ISO 17025 Helps Your Laboratory to Comply with the Regulation
Get your laboratory accredited to ISO standards. Use all tools that Zendo Lims offers you to help your laboratory with certification or accreditation, such as incidents management and document management.
Laboratories working with Zendo Lims, ISO have been successfully audited for accreditation in quality standards.ISO 17025, ISO 15189, ISO 9001 and even country-specific quality standards.
The process of the quality management system in the laboratory allows the laboratory to work according to the Good Practice Guidance (GLP) manual.
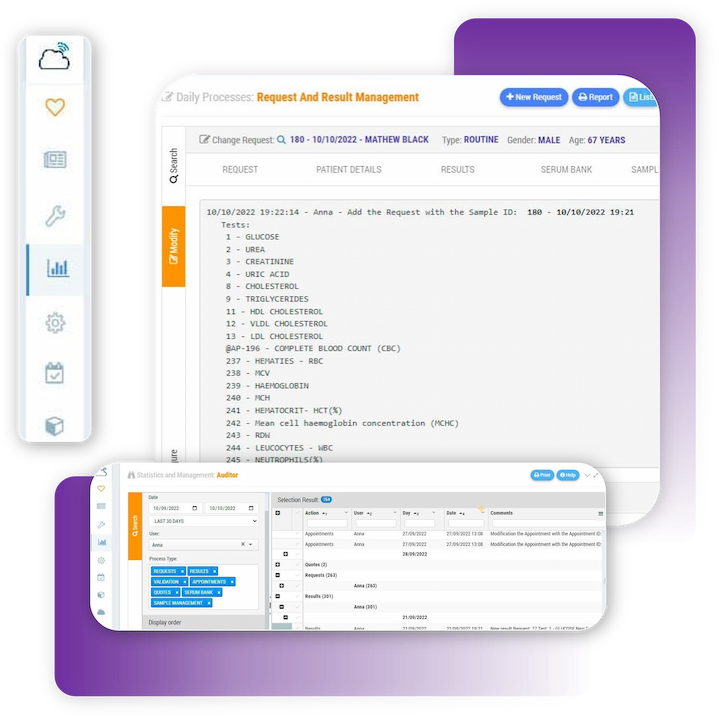
Zendo Lims is a Laboratory documents control software, it is therefore possible to attach all documentation concerning analytical procedures, analysers and measuring devices, customer and supplier contracts, etc.
Advanced Laboratory Policy Management Software for Standardization
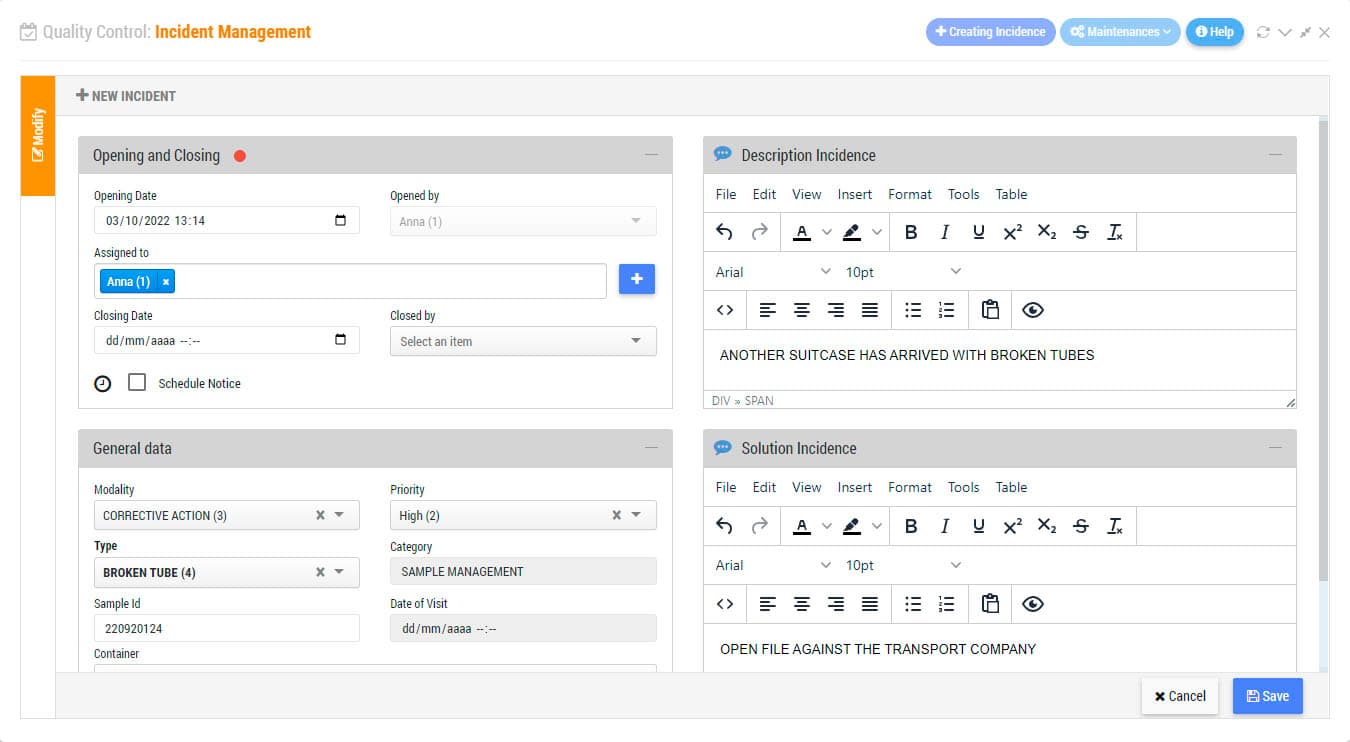
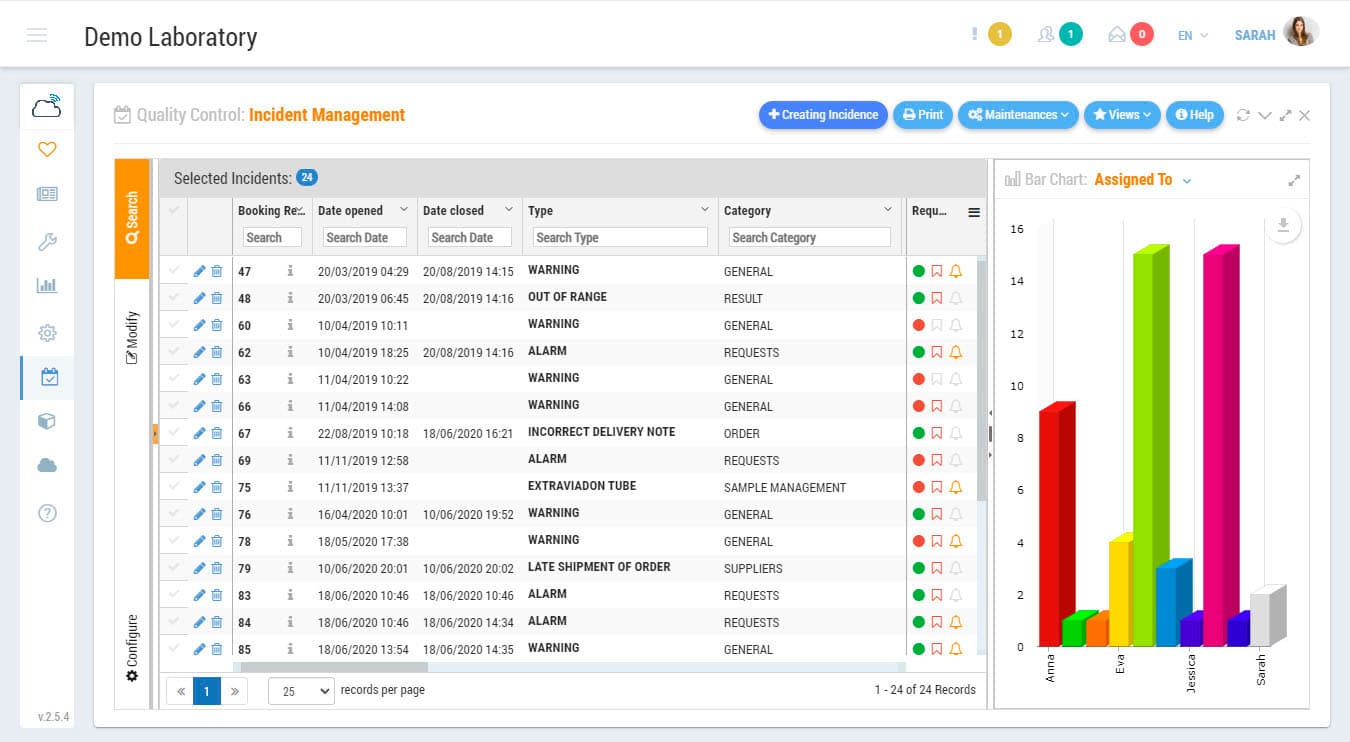
Incidents Management Module is an indispensable tool for the certification or accreditation of your laboratory to ISO Standards. Record non-conformities, corrective actions or preventive actions. Keep track of any abnormal situation in the laboratory. Define freely incidence categories to establish control points by sections and/or by types of laboratory activity. Indicate the level of priority, the assigned users, or establish a system of intelligent warnings for each of the incidents.
Zendo Lims allows integration with document management systems such as OnlyOffice, Microsoft Office 365 or Google G Suite and document repositories such as DropBox or Microsoft OneDrive. If you have a corporate document manager in the cloud, it is also possible to integrate it with Zendo Lims ISO 17025.
Reliable Lab Compliance Database Software for Your Laborator
LIMS 17025 Ready, that Enhances All Aspects of Your Laboratory
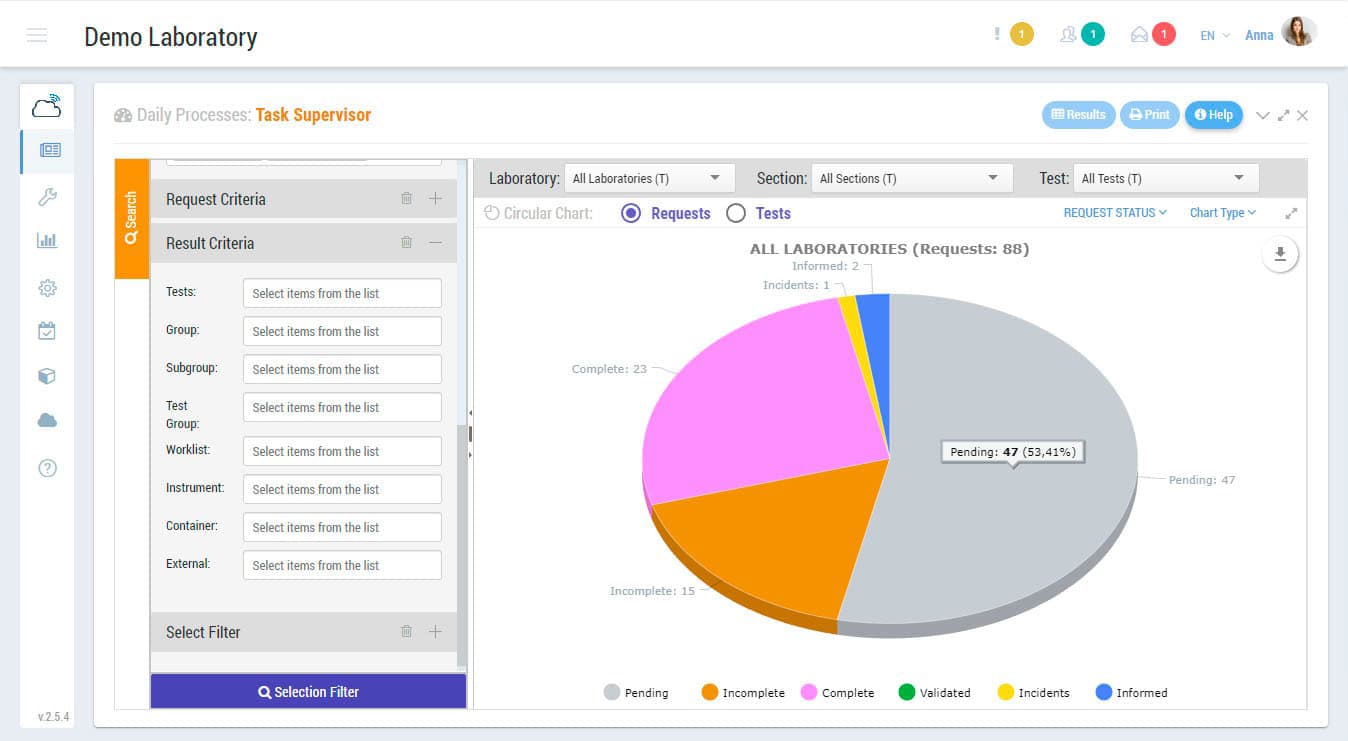
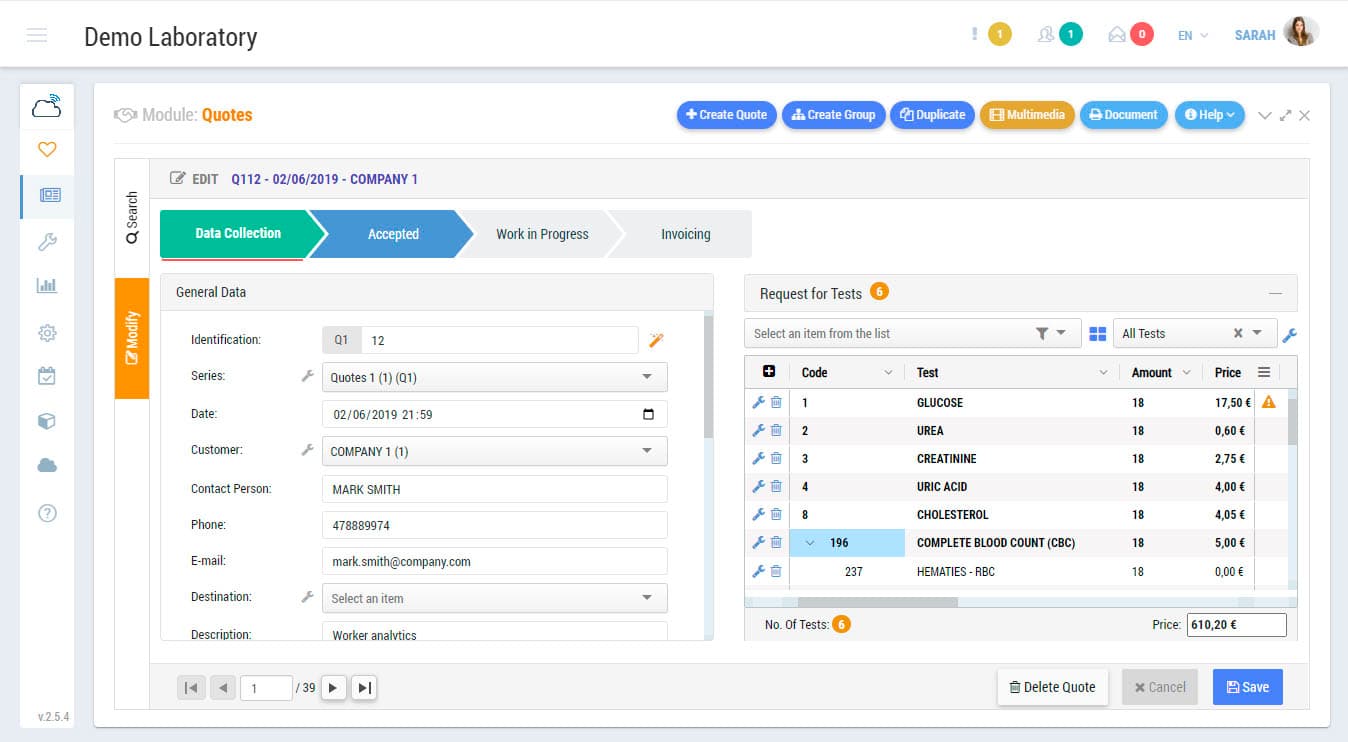
Sample management, results and validation, reports, connections with measuring devices, interconnections with external systems, invoicing, data mining, quality control, storage, etc. those are some of the functions that Zendo Lims, as 17025 software, makes available to you to guarantee the complete and integral management of the laboratory.
LIMS Tailored to Suit Every Sector and ISO Requirements
Move forward in the digital transformation of your laboratory by taking advantage of the benefits of working in the cloud. Zendo Lims flexibiliaty makes it easy to adapt.
Join Zendo Lims and experience the difference with our LIMS ISO System!
FAQs about our Lab Compliance Software
Zendo Lims ensures compliance with standard operating regulations by means of built-in features, such as:
Centralised regulations management which ensures that everyone works with the latest version. Allowing each document's version to be updated and controlled by the laboratory compliance software.
Access Control and Auditing . Zendo Lims, as a lab compliance database software or laboratory document management software, keeps a comprehensive and traceable logs of all activities carried out in the laboratory, which makes internal or external audits easier by proving regulatory compliance.
Compliance and continuous improvement : Zend Lims aligns with current quality standards such as ISO 17025, ISO 15189, GMP or GLP to assist the laboratory in its accreditation. Allowing you to identify areas for improvement, in order to make changes to your procedures to achieve continuous enhancement.
Integration with laboratory workflows to ensure that operating procedures are followed properly.